Ciclindole
 | |
| Clinical data | |
|---|---|
| Other names | WIN-27,147-2; WIN-27147-2; WIN27147-2 |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
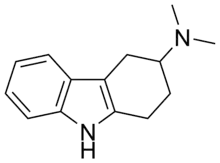
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H18N2 |
| Molar mass | 214.312 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Ciclindole (INNTooltip International Nonproprietary Name; developmental code name WIN-27,147-2), also known as cyclindole (USANTooltip United States Adopted Name), is an antipsychotic with a tricyclic and tryptamine-like structure that was never marketed.[1][2]
It displaces spiperone binding in vitro and elevates dopamine levels in the striatum, indicating that it acts as a dopamine D2 receptor antagonist.[2] It also shows apparent affinity for the α1-adrenergic receptor, the serotonin S1 receptor, and the serotonin S2 receptor.[2] However, its affinities for all of the preceding targets are weak, in the low micromolar range.[2]
The related drug flucindole is about 5 to 10 times more potent than ciclindole both in vitro and in vivo.[2]
See also
References
- ↑ Triggle DJ (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. ISBN 0-412-46630-9.
- 1 2 3 4 5 Wood PL, McQuade PS (1984). "Ciclindole and flucindole: novel tetrahydrocarbazolamine neuroleptics". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 8 (4–6): 773–7. doi:10.1016/0278-5846(84)90057-5. PMID 6152347. S2CID 39252411.
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Tryptamines |
|
|---|---|
| N-Acetyltryptamines |
|
| α-Alkyltryptamines |
|
| Triptans | |
| Cyclized tryptamines |
|
| Isotryptamines | |
| Related compounds |
|
| Classes |
|
|---|---|
| Antidepressants (Tricyclic antidepressants (TCAs)) |
|
| Antihistamines |
|
| Antipsychotics |
|
| Anticonvulsants | |
| Anticholinergics |
|
| Others |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.