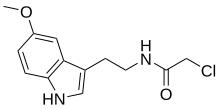
Isamide
 | |
| Clinical data | |
|---|---|
| Other names | N-(1-Chloroacetyl)-5-methoxytryptamine |
| Drug class | Serotonin receptor antagonist |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H15ClN2O2 |
| Molar mass | 266.73 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Isamide, also known as N-chloroacetyl-5-methoxytryptamine, is a serotonin receptor antagonist and the N-chloroacetyl derivative of 5-methoxytryptamine.[1][2][3] It was first described in the scientific literature by 1969 and was first pharmacologically characterized by 1979.[4][2]
References
- ↑ Frohlich PF, Meston CM (2000). "Evidence that serotonin affects female sexual functioning via peripheral mechanisms". Physiology & Behavior. 71 (3–4): 383–393. doi:10.1016/s0031-9384(00)00344-9. PMID 11150571.
- 1 2 Huidobro-Toro JP, Huidobro F, Ruiz M (June 1979). "N-Chloroacetyl 5-methoxytryptamine (isamide): a selective antagonist of 5-hydroxytryptamine in the rat uterus". The Journal of Pharmacy and Pharmacology. 31 (6): 371–374. doi:10.1111/j.2042-7158.1979.tb13525.x. PMID 39134.
- ↑ Huidobro-Toro JP, Foree B (February 1980). "Dual agonist-antagonist effects of 5-hydroxytryptamine (5-HT) in the guinea pig ileum: evidence for a selective receptor desensitization effect". European Journal of Pharmacology. 61 (4): 335–345. doi:10.1016/0014-2999(80)90072-2. PMID 6102916.
- ↑ Kobayashi T, Spande TF, Aoyagi H, Witkop B (July 1969). "Tricyclic analogs of melatonin". Journal of Medicinal Chemistry. 12 (4): 636–638. doi:10.1021/jm00304a017. PMID 5793155.
| Tryptamines |
|
|---|---|
| N-Acetyltryptamines |
|
| α-Alkyltryptamines |
|
| Triptans | |
| Cyclized tryptamines |
|
| Isotryptamines | |
| Related compounds |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.