FT-104
 | |
| Clinical data | |
|---|---|
| Other names | RE-104; RE104; 4-Glutaryloxy-N,N-diisopropyltryptamine; 4-Hydroxy-N,N-diisopropyltryptamine O-glutarate; O-Glutaryl-4-hydroxy-N,N-diisopropyltryptamine; 4-HO-DiPT glutarate; O-Glutaryl-4-HO-DiPT |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Duration of action | 3.7 hours[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
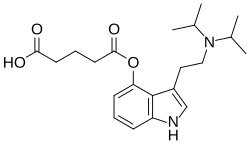
| Formula | C21H30N2O |
| Molar mass | 326.484 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
FT-104, also known as RE-104 and as 4-glutaryloxy-N,N-diisopropyltryptamine (4-HO-DiPT O-glutarate), is a psychedelic tryptamine derivative which is a prodrug ester of the well known designer drug 4-HO-DiPT.[2] It is one of a number of related derivatives developed for potential medical applications, and is in human clinical trials as a possible treatment for postpartum depression and treatment-resistant depression.[3][4][5][6]
It is being developed by the pharmaceutical company Reunion Neuroscience.[7] As of 2024, FT-104 is entering Phase II clinical trials for post-partum depression.[8]
Pharmacology
FT-104 is a prodrug that is converted into the psychedelic tryptamine 4-HO-DiPT upon administration.[2][9] 4-OH-DiPT then acts as an agonist on the serotonin 2A receptor which leads to an antidepressant effect.[7] Its duration in humans has been found to be 3.7 hours.[1]
Research
A clinical study of FT-104 for treatment of postpartum depression (PPD) has been published.[1]
See also
References
- 1 2 3 Pollack M, Hocevar-Trnka J, Bryson N, Taylor B, Johnson M, Alexander R (December 2024). "ACNP 63rd Annual Meeting: Poster Abstracts P609-P914: P697. RE104: A Novel, Shorter-Acting Psychedelic for Post Partum Depression". Neuropsychopharmacology. 49 (Suppl 1): 418–594 (469–470). doi:10.1038/s41386-024-02013-y. PMID 39643635.
- 1 2 Bryson N, Alexander R, Asnis-Alibozek A, Ehlers MD (June 2024). "RE104: Synthesis and Activity of a Novel Serotonergic Psychedelic Prodrug of 4-Hydroxy-N,N-diisopropyltryptamine". ACS Chemical Neuroscience. 15 (12): 2386–2395. doi:10.1021/acschemneuro.4c00058. PMC 11191588. PMID 38758589.
- ↑ Hallifax J (11 August 2022). "An Inside Look into Field Trip's Next-Generation Psychedelic, FT-104".
- ↑ WO 2022/000091, Bryson N, "Tryptamine prodrugs", published 6 January 2022, assigned to Field Trip Psychedelics Inc.
- ↑ US 2022/0024956, Slassi A, Araujo J, "Psilocin derivatives as serotonergic psychedelic agents for the treatment of CNS disorders.", published 27 January 2022, assigned to Mindset Pharma Inc.
- ↑ WO 2022/246572, Slassi A, Araujo J, Higgin GH, Gabriele J, "Hallucinogen-Fatty Acid Combination", published 1 December 2022, assigned to Mindset Pharma Inc.
- 1 2 Braner S (May 8, 2024). "Reunion Neuroscience raises $103 million for a psychedelic to treat depression". Chemical & Engineering News.
- ↑ Alexander R, Hocevar-Trnka J (June 26, 2024). "RE104: A Novel, Fast-Acting Psychedelic for Postpartum Depression". psychiatrictimes.com.
- ↑ "Reunion Neuroscience Announces Publication of Results from Early Preclinical Studies Demonstrating the Potential of RE104 for Development in Depressive Disorders". GlobalNewswire. May 20, 2024 – via Yahoo!Finance.