Cinanserin
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.220.552 |
| Chemical and physical data | |
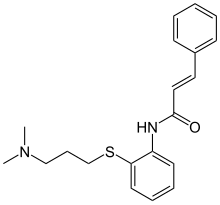
| Formula | C20H24N2OS |
| Molar mass | 340.49 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Cinanserin (INN) is a 5-HT2A and 5-HT2C receptor antagonist which was discovered in the 1960s.[1]
The molecule is an inhibitor of the 3C-like protease of SARS-CoV-1[2] and SARS-CoV-2.[3]
See also
References
- ↑ Neuman RS, Zebrowska G (December 1992). "Serotonin (5-HT2) receptor mediated enhancement of cortical unit activity". Canadian Journal of Physiology and Pharmacology. 70 (12): 1604–9. doi:10.1139/y92-230. PMID 1301238.
- ↑ Chen L, Gui C, Luo X, Yang Q, Günther S, Scandella E, et al. (June 2005). "Cinanserin is an inhibitor of the 3C-like proteinase of severe acute respiratory syndrome coronavirus and strongly reduces virus replication in vitro". Journal of Virology. 79 (11): 7095–103. doi:10.1128/JVI.79.11.7095-7103.2005. PMC 1112131. PMID 15890949.
- ↑ Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, Zhang B, Li X, Zhang L, Peng C, Duan Y, Yu J, Wang L, Yang K, Liu F, Jiang R, Yang X, You T, Liu X, Yang X, Bai F, Liu H, Liu X, Guddat LW, Xu W, Xiao G, Qin C, Shi Z, Jiang H, Rao Z, Yang H (June 2020). "Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors". Nature. 582 (7811): 289–293. doi:10.1038/s41586-020-2223-y. PMID 32272481.
Cinanserin is an inhibitor of SARS-CoV-2 Mpro.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.