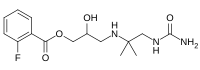
Flestolol
 | |
| Names | |
|---|---|
| IUPAC name
3-{[1-(Carbamoylamino)-2-methyl-2-propanyl]amino}-2-hydroxypropyl 2-fluorobenzoate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C15H22FN3O4 |
| Molar mass | 327.356 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Flestolol is a short-acting beta adrenergic receptor antagonist.[1]
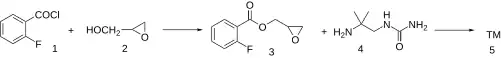
Synthesis
Acylation of acid chloride 2-Fluorobenzoyl chloride [393-52-2] (1) with glycidol (2) produces the ester 2,3-Epoxypropyl 2-Fluorobenzoate [85515-51-1] (3). Reaction of that intermediate with amine (2-Amino-2-methyl-propyl)-urea [87484-83-1] (4) obtained by reaction of 1,1-dimethylethylenediamine with urea, gives flestolol (5).
References
- ↑ Quon, CY; Stampfli, HF (1993). "Biochemical characterization of flestolol esterase". Research communications in chemical pathology and pharmacology. 81 (3): 309–22. PMID 8235065.
- ↑ Day, BW; Pento, JT; ACC-9089. Drugs Fut 1985, 10, 6, 447.
- ↑ Kam, Sheung Tsam; Matier, William L.; Mai, Khuong X.; Barcelon-Yang, Cynthia; Borgman, Robert J.; O'Donnell, John P.; Stampfli, Herman F.; Sum, Check Y.; Anderson, William G. (1984). "[(Arylcarbonyl)oxy]propanolamines. 1. Novel .beta.-blockers with ultrashort duration of action". Journal of Medicinal Chemistry. 27 (8): 1007. doi:10.1021/jm00374a013. PMID 6146718.
- ↑ Agustin Escobar, Dietmar M. Wagenknecht, Abu S. Alam, WO1985004581 (1985 to American Hospital Supply Corporation).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.