Pentylone
 | |
| Clinical data | |
|---|---|
| Other names | β-Keto-Methylbenzodioxolylpentanamine, βk-Methyl-K, βk-MBDP, methylenedioxypentedrone, 1‐(3,4‐methylenedioxyphenyl)‐2‐(methylamino)pentan‐1‐one[1] |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
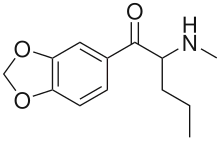
| Formula | C13H17NO3 |
| Molar mass | 235.283 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Pentylone is a stimulant developed in the 1960s.[3] It is a substituted cathinone that has been identified in some samples of powders sold as "NRG-1", along with varying blends of other cathinone derivatives including flephedrone, MDPBP, MDPV, and 4-MePPP. It was also found in combination with 4-MePPP being sold as "NRG-3".[1] Reports indicate side effects include paranoia, agitation, and insomnia, with effects lasting for several days at high doses.[4]
Pharmacology
Pentylone acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) and a serotonin releasing agent.[5]
Legality
Pentylone is banned in Canada, Germany, Sweden, the United States, and the United Kingdom.[6][7]
See also
References
- 1 2 Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J (September 2011). "Analysis of NRG 'legal highs' in the UK: identification and formation of novel cathinones". Drug Testing and Analysis. 3 (9): 569–575. doi:10.1002/dta.204. PMID 21960541.
- ↑ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ↑ GB 1085135, "Substituted phenyl-α-amino ketones.", issued 1969
- ↑ Bish J (4 August 2017). "Watch Out for Pentylone, the Horrible New MDMA Additive". Vice.
- ↑ Simmler LD, Rickli A, Hoener MC, Liechti ME (April 2014). "Monoamine transporter and receptor interaction profiles of a new series of designer cathinones". Neuropharmacology. 79: 152–160. doi:10.1016/j.neuropharm.2013.11.008. PMID 24275046. S2CID 25259854.
- ↑ "Cannabinoider föreslås bli klassade som hälsofarlig vara" [Cannabinoids are proposed to be classified as a health hazard]. Folkhalsomyndigheten [Public Health Agency of Sweden] (in Swedish). Archived from the original on 25 March 2015. Retrieved 29 June 2015.
- ↑ "Schedules of Controlled Substances: Temporary Placement of 10 Synthetic Cathinones Into Schedule I". Drug Enforcement Administration. U.S. Federal Register. 7 March 2014.
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.