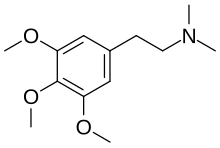
Trichocereine
 | |
| Names | |
|---|---|
| IUPAC name
N,N-dimethyl-2-(3,4,5-trimethoxyphenyl)ethanamine | |
| Other names
N,N-Dimethyl-3,4,5-trimethoxyphenethylamine; N,N-Dimethylmescaline; 3,4,5-Trimethoxy-N,N-dimethylbenzeneethanamine; MM-M | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C13H21NO3 |
| Molar mass | 239.31 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Trichocereine, also known as N,N-dimethyl-3,4,5-trimethoxyphenethylamine or as N,N-dimethylmescaline (MM-M), is a phenethylamine alkaloid that is found in several plant species and is closely related to mescaline (3,4,5-trimethoxyphenethylamine).[1][2] It was first reported in the Trichocereus terscheckii cactus in 1935 and was subsequently isolated from Gymnocalycium spp. and Turbinicarpus spp. cacti.[1][2] Additionally, it has been found in the shrubs Acacia berlandieri and Acacia rigidula.[1] In contrast to mescaline, trichocereine has been found to lack psychoactive effects in humans at doses of up to 550 mg.[1][2] Similarly, the compound showed no activity in the conditioned avoidance test in rodents.[1][3]
See also
- N-Methylmescaline
- 3-Hydroxy-N,N-dimethylphenethylamine (LSM-6)
References
- 1 2 3 4 5 Shulgin A, Manning T, Daley PF (2011). "#125. Trichocereine". The Shulgin Index, Volume One: Psychedelic Phenethylamines and Related Compounds. Vol. 1. Berkeley, CA: Transform Press. pp. 309–310. ISBN 978-0-9630096-3-0. OCLC 709667010.
- 1 2 3 Luduena, F.P. (1935) Pharmacology of trichocereine, an alkaloid from the cactus Trichocereus terscheki (Parm.) Britton and Rose. Revista de la Sociedad Argentina de Biologia 11: 604–610.
- ↑ Browne RG, Harris RT, Ho BT (1974). "Stimulus properties of mescaline and N-methylated derivatives: difference in peripheral and direct central administration". Psychopharmacologia. 39 (1): 43–56. doi:10.1007/BF00421457. PMID 4425137.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.