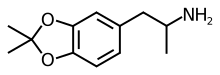
3,4-Isopropylidenedioxyamphetamine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | IDA; Isopropylidenedioxyamphetamine |
| Drug class | Monoamine releasing agent; Entactogen; Serotonergic psychedelic; Hallucinogen |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H17NO2 |
| Molar mass | 207.273 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
3,4-Isopropylidenedioxyamphetamine (IDA) is a monoamine releasing agent (MRA) of the amphetamine family related to 3,4-methylenedioxyamphetamine (MDA).[1][2][3] It is considerably less potent than MDA as an MRA in vitro.[3][1] IDA fully substituted for MDMA and LSD in animal drug discrimination tests, albeit with 5- to 7-fold lower potency than MDA.[3][1]
See also
- 3,4-Ethylidenedioxyamphetamine (EIDA)
- 3,4-Ethylenedioxyamphetamine (EDA)
- 3,4-Ethylenedioxymethamphetamine (EDMA)
- 3,4-Ethylenedioxymethcathinone (EDMC)
References
- 1 2 3 Nichols DE, Oberlender R, Burris K, Hoffman AJ, Johnson MP (November 1989). "Studies of dioxole ring substituted 3,4-methylenedioxyamphetamine (MDA) analogues". Pharmacol Biochem Behav. 34 (3): 571–576. doi:10.1016/0091-3057(89)90560-1. PMID 2623014.
- ↑ Heal DJ, Gosden J, Smith SL (November 2018). "Evaluating the abuse potential of psychedelic drugs as part of the safety pharmacology assessment for medical use in humans". Neuropharmacology. 142: 89–115. doi:10.1016/j.neuropharm.2018.01.049. PMID 29427652.
- 1 2 3 Sáez-Briones P, Hernández A (September 2013). "MDMA (3,4-Methylenedioxymethamphetamine) Analogues as Tools to Characterize MDMA-Like Effects: An Approach to Understand Entactogen Pharmacology". Curr Neuropharmacol. 11 (5): 521–534. doi:10.2174/1570159X11311050007. PMC 3763760. PMID 24403876.
Additionally, EDA (ethyl[idene]dioxyamphetamine) has been demonstrated to be nearly equipotent to MDA in its ability to induce [3H]5-HT and [3H]dopamine release from rat hippocampal slices, whereas IDA (isopropylidenedioxyamphetamine) was considerably less potent [114]. In drug discrimination experiments, complete substitution for LSD and MDMA was found for EDA and IDA, which also correlates in the latter case with [125I]DOI displacement.
| Phenylalkyl- amines (other than cathinones) |
|
|---|---|
| Cyclized phenyl- alkylamines | |
| Cathinones |
|
| Tryptamines | |
| Chemical classes | |
| DRAsTooltip Dopamine releasing agents |
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRAsTooltip Norepinephrine releasing agents |
| ||||||||||||||
| SRAsTooltip Serotonin releasing agents |
| ||||||||||||||
| Others |
| ||||||||||||||
See also: Receptor/signaling modulators • Monoamine reuptake inhibitors • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins | |||||||||||||||
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.