The fire would look like a normal fire. However, the smoke would do weird things.
Here is a diagram of the O'Neill cylinder with a fire. (Darker blue represents higher air pressure.)
The radius of the smoke column will grow more than on earth as the altitude increases, since the centrifugal force decreases as the smoke reaches the inner part of the cylinder.
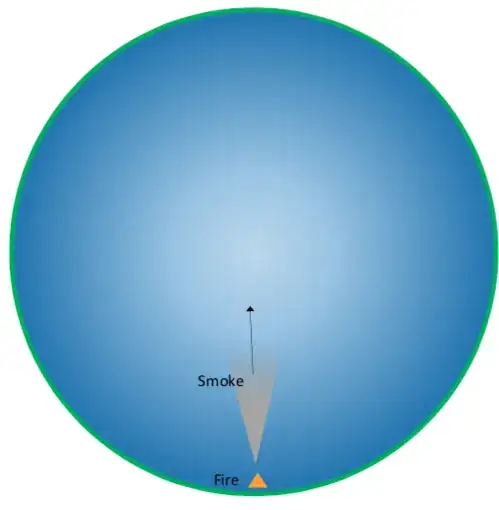
At this point, it looks normal. Then, it gets weird.
As the smoke reaches lower air pressure, it cannot rise because it is mostly composed of solids and liquids, and solids and liquids do not expand. The smoke will stay in the area where its density equals the density of the air, beginning to form a cylinder.

The new, high temperature smoke will displace the older smoke, pushing it down the O'Neill cylinder. Particles of smoke are held up by their high temperature. Since the cylinder of smoke is constantly losing temperature as it leaves the fire, the cylinder of smoke will undergo dry precipitation, "sinking" back to the edge of the O'Neill cylinder.

I hope my pictures helped to portray what I was saying. Please comment if you are confused.


