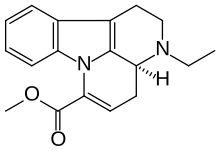
Vinconate
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.067.969 |
| Chemical and physical data | |
| Formula | C18H20N2O2 |
| Molar mass | 296.370 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Vinconate is a synthetic vincamine analog used as a nootropic.[1]
Vinconate, even when systemically administered, enhances the endogenous release of dopamine in the striatum, probably via the stimulation of presynaptic muscarinic receptors.[2]
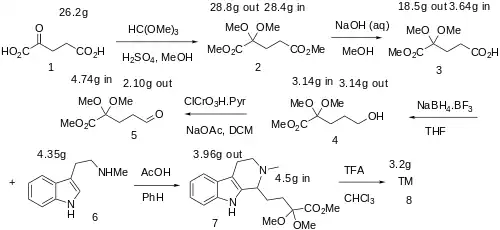
Synthesis
References
- ↑ Brimble MA, Levi MS (2010). "A Review of Neuroprotective Agents". In Atta-ur-Rahman, Reitz AB (eds.). Frontiers in Medicinal Chemistry. Vol. 3. SAIF Zone: Bentham Science Publishers. p. 182. ISBN 978-1-60805-206-6.
- ↑ Iino T, Katsura M, Kuriyama K. Effect of vinconate on the extracellular levels of dopamine and its metabolites in the rat striatum: microdialysis studies. Eur J Pharmacol. 1995 Nov 3;286(1):99-103. doi: 10.1016/0014-2999(95)00545-v. PMID: 8566157.
- ↑ Castaer, J.; Serradell, MN; Vinconate. Drugs Fut 1984, 9, 4, 283.
- ↑ Jean A. A. J. Hannart, U.S. patent 4,200,638 (1980 to Omnium Chimique SA).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.