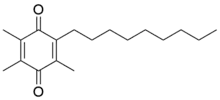
Utreloxastat
 | |
| Clinical data | |
|---|---|
| Other names | PTC857 |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H28O2 |
| Molar mass | 276.420 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Utreloxastat (PTC857) is an investigational new drug developed by PTC Therapeutics for the treatment of amyotrophic lateral sclerosis (ALS).[1]
Pharmacology
Utreloxastat is a 15-lipoxygenase (15-LO) inhibitor that has been used to study α-synucleinopathies, tauopathies, traumatic brain injury, and ischemic-reperfusion related injuries. Utreloxastat is a redox‐active inhibitor of ferroptosis.[2]
Clinical trials
Utreloxastat has reached Phase 2 clinical trials for the treatment of amyotrophic lateral sclerosis (ALS).[3][4][5] However this trial failed to show significant efficacy in slowing disease progression.[6]
See also
References
- ↑ "Utreloxastat - PTC Therapeutics". AdisInsight. Springer Nature Switzerland AG.
- ↑ "Utreloxastat". MedChemExpress.
- ↑ Gao L, Giannousis P, Thoolen M, Kaushik D, Latham J, Tansy A, et al. (February 2023). "First-in-Human Studies of Pharmacokinetics and Safety of Utreloxastat (PTC857), a Novel 15-Lipooxygenase Inhibitor for the Treatment of Amyotrophic Lateral Sclerosis". Clinical Pharmacology in Drug Development. 12 (2): 141–151. doi:10.1002/cpdd.1203. PMC 10107758. PMID 36516010.
- ↑ Katz JS, Vu T, Chio A, McDermott C, Devos D, Johnston M, et al. (2024-04-14). "CARDINALS: A Phase 2, Randomized, Double-blind, Placebo-controlled, Parallel-group Study to Evaluate the Efficacy and Safety of Utreloxastat (PTC857) in Patients with Amyotrophic Lateral Sclerosis (P5-11.010)". Neurology. 102 (17_supplement_1): 3593. doi:10.1212/WNL.0000000000205241.
- ↑ Li X, Bedlack R (June 2024). "Evaluating emerging drugs in phase II & III for the treatment of amyotrophic lateral sclerosis". Expert Opinion on Emerging Drugs. 29 (2): 93–102. doi:10.1080/14728214.2024.2333420. PMID 38516735.
- ↑ "PTC Therapeutics Announces Topline Results of CardinALS Trial of Utreloxastat in ALS Patients". PTC Therapeutics, Inc. 26 November 2024.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.