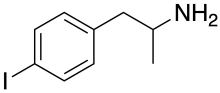
Para-Iodoamphetamine
 | |
| Clinical data | |
|---|---|
| Drug class | Serotonin releasing agent; Serotonergic neurotoxin |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C9H12IN |
| Molar mass | 261.106 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
para-Iodoamphetamine (PIA), also known as 4-iodoamphetamine (4-IA), is a monoamine releasing agent (MRA) and serotonergic neurotoxin of the amphetamine family related to para-chloroamphetamine (PCA).[1]
Pharmacology
Pharmacodynamics
PIA acts as a serotonin releasing agent (SRA).[2] In animal drug discrimination tests, PIA fully substitutes for MDMA and (+)-MBDB.[1]
PIA has been described as having either similar serotonergic neurotoxicity as PCA[1] or as having much weaker serotonergic neurotoxicity than PCA.[2][3]
Chemistry
PIA, also known as 4-iodoamphetamine, is a phenethylamine and amphetamine derivative and a para-halogenated amphetamine.
Analogues
PIA is closely related to other para-halogenated amphetamines such as PCA, para-bromoamphetamine (PBA), and para-fluoroamphetamine (PFA).
Iofetamine, also known as N-isopropyl-(123I)-para-iodoamphetamine, is a derivative of PIA used as a radiopharmaceutical and diagnostic agent.[4]
5-Iodo-2-aminoindane (5-IAI), the 2-aminoindane analogue of PIA, was an attempt to make a non-neurotoxic analogue of PIA that proved to be less neurotoxic.[1]
References
- 1 2 3 4 Nichols DE, Marona-Lewicka D, Huang X, Johnson MP (1993). "Novel serotonergic agents". Drug des Discov. 9 (3–4): 299–312. PMID 8400010.
- 1 2 Marona-Lewicka D, Rhee GS, Sprague JE, Nichols DE (December 1995). "Psychostimulant-like effects of p-fluoroamphetamine in the rat". European Journal of Pharmacology. 287 (2): 105–113. doi:10.1016/0014-2999(95)00478-5. PMID 8749023.
- ↑ Nichols DE, Johnson MP, Oberlender R (January 1991). "5-Iodo-2-aminoindan, a nonneurotoxic analogue of p-iodoamphetamine". Pharmacology, Biochemistry, and Behavior. 38 (1): 135–139. CiteSeerX 10.1.1.670.504. doi:10.1016/0091-3057(91)90601-W. PMID 1826785. S2CID 20485505.
- ↑ Druckenbrod RW, Williams CC, Gelfand MJ (January 1989). "Iofetamine hydrochloride I 123: a new radiopharmaceutical for cerebral perfusion imaging". DICP. 23 (1): 19–24. doi:10.1177/106002808902300103. PMID 2655294.