Lumefantrine
 | |
| Clinical data | |
|---|---|
| Other names | benflumetol |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a609024 |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 99.7% |
| Metabolites | desbutyl-lumefantrine |
| Elimination half-life | 3-6 days |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.133.797 |
| Chemical and physical data | |
| Formula | C30H32Cl3NO |
| Molar mass | 528.94 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 130 to 132 °C (266 to 270 °F) |
| Solubility in water | 30.9ng/mL |
SMILES
| |
InChI
| |
| | |
Lumefantrine (or benflumetol) is an antimalarial drug. It is only used in combination with artemether. The term "co-artemether" is sometimes used to describe this combination.[1] Lumefantrine has a much longer half-life compared to artemether (3-6 days vs. 2 hours[2]), and is therefore thought to clear any residual parasites that remain after combination treatment.[3]
Mechanism of action
Exact mechanism by which lumefantrine acts on erythrocytic stages of Plasmodium falciparum is unknown. However, it was shown to exert its action through possible two mechanisms:[3][2][4]
- inhibiting β-hematin formation by creating complexes with hemin
- inhibiting nucleic acid and protein synthesis
Moreover, it was shown to interact with human sodium/potassium ATPase subunit α1.[5]
Metabolism
Lumefantrine is metabolised in the liver by cytochrome P450 3A4 isoenzyme (CYP3A4) and 2D6 (CYP2D6), yielding desbutyl-lumefantrine as a major metabolite.[5][2]
Adverse effects
Lumefantrine, as used in combination with artemether, was shown to induce the following side effects:
- prolongation of QT interval, especially in combination with other drugs exhibiting the same effects or in patients with congenital prolongation of the QT interval
- hypersensitivity reactions
- interactions with CYP3A4 and CYP2D6 inducing or inhibiting drugs
- infertility (sperm abnormalities and trouble getting pregnant)
People taking efavirenz as a part of HIV therapy should be wary of potential deviations during treatment, due to a decrease of AUC of this antiretroviral.[6][7]
History
Lumefantrine, along with pyronaridine and naphthoquine, were synthesized during the Chinese Project 523 antimalaria drug research effort initiated in 1967; these compounds are all used in combination antimalaria therapies.[8][9][10]
Research
Lumefantrine is being investigated as a part of a regimen with ganaplacide for the treatment of Plasmodium falciparum malaria.[11]
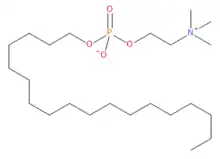
Along with O-choline (octadecyl 2-(trimethylammonio)ethyl phosphate), lumefantrine inhibits in vivo growth of Theileria equi and Babesia caballi, due to inhibition of membrane phospholipid synthesis, hemoglobin digestion and targeting lactate metabolism.[12] Additionally, it can inhibit Babesia gibsoni growth in vitro (synergistically with artemisinin derivatives).[13]
It may exert negative effects on aquatic ecosystems by adversely acting on Chlorella vulgaris, Raphidocelis subcapitata, Lemna minor and Microcystis aeruginosa.[14][15] Moreover, it is classified as a potential endocrine disrupting compound by decreasing FSHB and increasing prolactin secretion.[16][17]
Lumefantrine and calcium phosphate-loaded lipid nanoparticles or cubosomes were investigated as a potential treatment of lung cancer due to probable antiangiogenic and anti-inflammatory properties of this combination.[18][19]
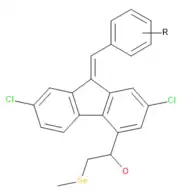
Selene-containing lumefantrine derivatives synthesised through Knoevenagel condensation (which itself is used to synthesise lumefantrine) exhibit potential antibacterial and antifungal activity. Compared with ciprofloxacin, they were shown to more potently bind to E. coli MurB enzyme – an enzyme participating in cell cycle and cell wall synthesis.[21]
See also
References
- ↑ Toovey S, Jamieson A, Nettleton G (August 2003). "Successful co-artemether (artemether-lumefantrine) clearance of falciparum malaria in a patient with severe cholera in Mozambique". Travel Medicine and Infectious Disease. 1 (3): 177–179. doi:10.1016/j.tmaid.2003.09.002. PMID 17291911.
- 1 2 3 "Coartem - Highlights of prescribing information" (PDF). Novartis. August 2019. Retrieved 26 February 2025.
{{cite web}}: CS1 maint: url-status (link) - 1 2 White NJ, van Vugt M, Ezzet F (August 1999). "Clinical pharmacokinetics and pharmacodynamics and pharmacodynamics of artemether-lumefantrine". Clinical Pharmacokinetics. 37 (2): 105–125. doi:10.2165/00003088-199937020-00002. PMID 10496300. S2CID 72714420.
- ↑ "Lumefantrine". go.drugbank.com. Retrieved 2025-02-26.
- 1 2 "Lumefantrine". go.drugbank.com. Retrieved 2025-02-26.
- ↑ Usman, Sikiru Olatunji; Oreagba, Ibrahim Adekunle; Akinyede, Akinwumi Akinyinka; Agbaje, Esther Oluwatoyin; Akinleye, Moshood Olusola; Onwujuobi, Adaobi Goodness; Ken-Owotor, Chioma; Adeuja, Olatunbosun; Ogunfowokan, Tosin; Kogbe, Segun; Owolabi, Emmanuel Tope; Adeniji, Hannah; Busari, Abdul Wasiu; Hassan, Olayinka Olayiwola; Abideen, Ganiu (2020-11-01). "Effect of nevirapine, efavirenz and lopinavir/ritonavir on the therapeutic concentration and toxicity of lumefantrine in people living with HIV at Lagos University Teaching Hospital, Nigeria". Journal of Pharmacological Sciences. 144 (3): 95–101. doi:10.1016/j.jphs.2020.07.013. ISSN 1347-8613.
- ↑ Zakaria, Zaril; Badhan, Raj K. S. (2018-07-01). "The impact of CYP2B6 polymorphisms on the interactions of efavirenz with lumefantrine: Implications for paediatric antimalarial therapy". European Journal of Pharmaceutical Sciences. 119: 90–101. doi:10.1016/j.ejps.2018.04.012. ISSN 0928-0987.
- ↑ Cui L, Su XZ (October 2009). "Discovery, mechanisms of action and combination therapy of artemisinin". Expert Review of Anti-Infective Therapy. 7 (8): 999–1013. doi:10.1586/eri.09.68. PMC 2778258. PMID 19803708.
- ↑ Benjamin J, Moore B, Lee ST, Senn M, Griffin S, Lautu D, et al. (May 2012). "Artemisinin-naphthoquine combination therapy for uncomplicated pediatric malaria: a tolerability, safety, and preliminary efficacy study". Antimicrobial Agents and Chemotherapy. 56 (5): 2465–2471. doi:10.1128/AAC.06248-11. PMC 3346652. PMID 22330921.
- ↑ Laman M, Moore BR, Benjamin JM, Yadi G, Bona C, Warrel J, et al. (December 2014). "Artemisinin-naphthoquine versus artemether-lumefantrine for uncomplicated malaria in Papua New Guinean children: an open-label randomized trial". PLOS Medicine. 11 (12): e1001773. doi:10.1371/journal.pmed.1001773. PMC 4280121. PMID 25549086.
- ↑ Ogutu, Bernhards; Yeka, Adoke; Kusemererwa, Sylvia; Thompson, Ricardo; Tinto, Halidou; Toure, Andre Offianan; Uthaisin, Chirapong; Verma, Amar; Kibuuka, Afizi; Lingani, Moussa; Lourenço, Carlos; Mombo-Ngoma, Ghyslain; Nduba, Videlis; N'Guessan, Tiacoh Landry; Nassa, Guétawendé Job Wilfried (2023-09-01). "Ganaplacide (KAF156) plus lumefantrine solid dispersion formulation combination for uncomplicated Plasmodium falciparum malaria: an open-label, multicentre, parallel-group, randomised, controlled, phase 2 trial". The Lancet Infectious Diseases. 23 (9): 1051–1061. doi:10.1016/S1473-3099(23)00209-8. ISSN 1473-3099. PMID 37327809.
- ↑ Maji, Chinmoy; Goel, Praveen; Suthar, A.; Mandal, Kruti D.; Gopalakrishnan, A.; Kumar, Rajender; Tripathi, B. N.; Kumar, Sanjay (2019-04-01). "Lumefantrine and o-choline – Parasite metabolism specific drug molecules inhibited in vitro growth of Theileria equi and Babesia caballi in MASP culture system". Ticks and Tick-borne Diseases. 10 (3): 568–574. doi:10.1016/j.ttbdis.2019.01.004. ISSN 1877-959X.
- ↑ Iguchi, Aiko; Matsuu, Aya; Matsuyama, Kazuyoshi; Hikasa, Yoshiaki (2015-04-01). "The efficacy of artemisinin, artemether, and lumefantrine against Babesia gibsoni in vitro". Parasitology International. 64 (2): 190–193. doi:10.1016/j.parint.2014.12.006. ISSN 1383-5769.
- ↑ Chia, Mathias Ahii; Ameh, Ilu; Agee, Jerry Tersoo; Otogo, Regina Anya; Shaba, Ahmad Fatima; Bashir, Hadiza; Umar, Fatima; Yisa, Abraham Gana; Uyovbisere, Ejiroghene Ebelechukwu; Sha’aba, Ramatu Idris (2021-07-01). "Effects of the antimalarial lumefantrine on Lemna minor, Raphidocelis subcapitata and Chlorella vulgaris". Environmental Toxicology and Pharmacology. 85: 103635. doi:10.1016/j.etap.2021.103635. ISSN 1382-6689.
- ↑ Dauda, Suleiman; Uyovbisere, Ejiroghene Ebelechukwu; Alhassan, Abdullahi Bala; Sha’aba, Ramatu Idris; Gadzama, Ibrahim Madu Katsallah; Onaji, Maria Onma; Chia, Mathias Ahii (2024-05-01). "Allelopathic interactions between Lemna minor and Microcystis aeruginosa are influenced by the antimalarial drug lumefantrine". Aquatic Botany. 192: 103759. doi:10.1016/j.aquabot.2024.103759. ISSN 0304-3770.
- ↑ Andres, Sandrine; Dulio, Valeria (2024-04-08), S109 | PARCEDC | List of 7074 potential endocrine disrupting compounds (EDCs) by PARC T4.2, Hiba Mohammed Taha, Zenodo, doi:10.5281/ZENODO.10944198, retrieved 2025-02-26
- ↑ Abolaji, Ao; Adesanoye, Oa; Awogbindin, I; Farombi, Eo (November 2016). "Endocrine disruption and oxidative stress implications of artemether–lumefantrine combination therapy in the ovary and uterus of rats". Human & Experimental Toxicology. 35 (11): 1173–1182. doi:10.1177/0960327115626580. ISSN 0960-3271.
- ↑ Sethuraman, Vaidevi; Janakiraman, Kumar; Krishnaswami, Venkateshwaran; Natesan, Subramanian; Kandasamy, Ruckmani (2021-03-01). "In vivo synergistic anti-tumor effect of lumefantrine combined with pH responsive behavior of nano calcium phosphate based lipid nanoparticles on lung cancer". European Journal of Pharmaceutical Sciences. 158: 105657. doi:10.1016/j.ejps.2020.105657. ISSN 0928-0987.
- ↑ Sethuraman, Vaidevi; Janakiraman, Kumar; Krishnaswami, Venkateshwaran; Natesan, Subramanian; Kandasamy, Ruckmani (2019-11-01). "pH responsive delivery of lumefantrine with calcium phosphate nanoparticles loaded lipidic cubosomes for the site specific treatment of lung cancer". Chemistry and Physics of Lipids. Practical insights into drug delivery systems. 224: 104763. doi:10.1016/j.chemphyslip.2019.03.016. ISSN 0009-3084.
- ↑ Puthran, Divyaraj; Poojary, Boja; Nayak, Soukhyarani Gopal; Purushotham, Nikil; Rasheed, Mohammed Shafeeulla; Hegde, Hemant (2020). "Design, synthesis, molecular docking, and biological evaluation of novel selenium containing lumefantrine analogues". Journal of Heterocyclic Chemistry. 57 (3): 1319–1329. doi:10.1002/jhet.3868. ISSN 1943-5193.
- ↑ Puthran, Divyaraj; Poojary, Boja; Nayak, Soukhyarani Gopal; Purushotham, Nikil; Rasheed, Mohammed Shafeeulla; Hegde, Hemant (2020). "Design, synthesis, molecular docking, and biological evaluation of novel selenium containing lumefantrine analogues". Journal of Heterocyclic Chemistry. 57 (3): 1319–1329. doi:10.1002/jhet.3868. ISSN 1943-5193.