Desmethylzopiclone
 | |
| Names | |
|---|---|
| IUPAC name
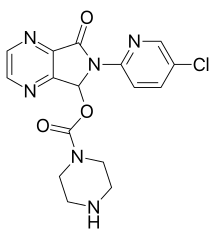
[6-(5-Chloropyridin-2-yl)-5-oxo-7H-pyrrolo[3,4-b]pyrazin-7-yl] piperazine-1-carboxylate | |
| Other names
SEP-174559 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C16H15ClN6O3 |
| Molar mass | 374.79 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Desmethylzopiclone, also known as SEP-174559, is an active metabolite of the sedative-hypnotic drug zopiclone.
Pharmacology
Unlike its parent compound, which is a largely non-selective benzodiazepine receptor agonist, desmethylzopiclone is a selective partial agonist at the benzodiazepine site of α3-containing GABA receptor subtypes.[1] It is also an antagonist to nicotinic acetylcholine receptors and NMDA receptors.[1]
Desmethylzopiclone has been described as a potential anxio-selective metabolite of zolpiclone owing to its selective affinity for the modulation of α3-containing GABA receptor subtypes.[1] Modulation of these GABAA subtypes have been implicated as key mediators of the anxiolytic effects of benzodiazepines.[2]
In forensic analysis
A method for the quantification of desmethylzopiclone from urine has been developed and may serve useful in forensic analysis of cases involving zolpiclone intoxication.[3]
References
- 1 2 3 Fleck, Mark W. (2002-08-01). "Molecular Actions of (S)-Desmethylzopiclone (SEP-174559), an Anxiolytic Metabolite of Zopiclone". Journal of Pharmacology and Experimental Therapeutics. 302 (2): 612–618. doi:10.1124/jpet.102.033886. ISSN 0022-3565. PMID 12130723.
- ↑ Rowlett, James K.; Platt, Donna M.; Lelas, Snjezana; Atack, John R.; Dawson, Gerard R. (2005-01-18). "Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates". Proceedings of the National Academy of Sciences. 102 (3): 915–920. Bibcode:2005PNAS..102..915R. doi:10.1073/pnas.0405621102. ISSN 0027-8424. PMC 545524. PMID 15644443.
- ↑ Nilsson, Gunnel H.; Kugelberg, Fredrik C.; Ahlner, Johan; Kronstrand, Robert (2014). "Quantitative Analysis of Zopiclone, N-desmethylzopiclone, Zopiclone N-oxide and 2-Amino-5-chloropyridine in Urine Using LC–MS-MS". Journal of Analytical Toxicology. 38 (6): 327–334. doi:10.1093/jat/bku042. PMID 24790062.