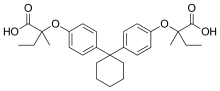
Clinofibrate
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H36O6 |
| Molar mass | 468.590 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Clinofibrate (INN) (trade name Lipoclin) is a fibrate and a derivative of Bisphenol Z.[1]
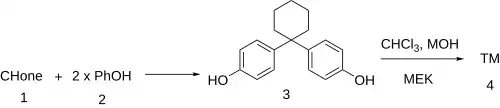
Synthesis
The reaction between cyclohexanone and phenol gives Bisphenol Z (3).[2] This is treated with chloroform and methyl ethyl ketone in the presence of base. The resulting Bargellini reaction gives clinofibrate.[3]
References
- ↑ Takeuchi N, Kukita H, Kajiyama G, Fujiyama M, Ishikawa K, Miki H, Mishima T, Murata K, Asano T (April 1982). "Effect of clinofibrate, a new hypolipidemic agent, on biliary and serum lipids in patients with hyperlipidemia". Atherosclerosis. 42 (2–3): 129–39. doi:10.1016/0021-9150(82)90145-9. PMID 7073798.
- ↑ Patil LS, Suryawanshi VS, Pawar OB, Shinde ND (2011). "An Improved, Highly Efficient Method for the Synthesis of Bisphenols". e-Journal of Chemistry. 8 (4): 2016–2019. doi:10.1155/2011/372673.
- ↑ US 3716583, Nakamura Y, Aono S, Agatsuma K, Tanaka Y, "Phenoxy carboxylic acid derivative", issued 13 February 1973, assigned to Sumitomo Chemical Company
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.