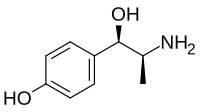
P-Hydroxynorephedrine
 | |
| Clinical data | |
|---|---|
| Other names | 4-Hydroxynorephedrine para-Hydroxynorephedrine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C9H13NO2 |
| Molar mass | 167.208 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
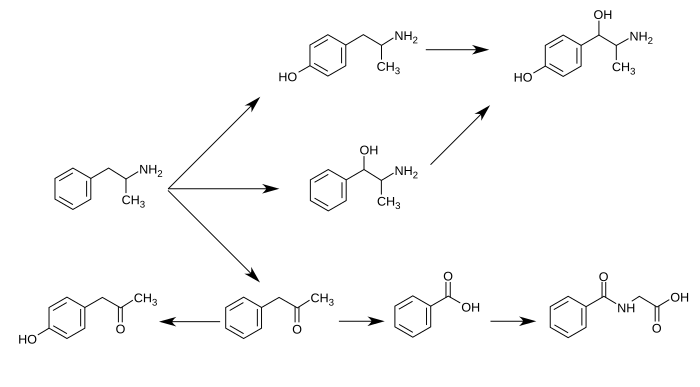
p-Hydroxynorephedrine (PHN or 4-hydroxynorephedrine) is the para-hydroxy analog of norephedrine and an active sympathomimetic metabolite of amphetamine in humans.[1][2] When it occurs as a metabolite of amphetamine, it is produced from both p-hydroxyamphetamine and norephedrine.[2][3][4]
Amphetamine metabolism
Metabolic pathways of amphetamine in humans[sources 1]
|
Notes
- ↑ 4-Hydroxyamphetamine has been shown to be metabolized into 4-hydroxynorephedrine by dopamine beta-hydroxylase (DBH) in vitro and it is presumed to be metabolized similarly in vivo.'"`UNIQ--ref-00000032-QINU`"''"`UNIQ--ref-00000033-QINU`"' Evidence from studies that measured the effect of serum DBH concentrations on 4-hydroxyamphetamine metabolism in humans suggests that a different enzyme may mediate the conversion of 4-hydroxyamphetamine to 4-hydroxynorephedrine;'"`UNIQ--ref-00000034-QINU`"''"`UNIQ--ref-00000035-QINU`"' however, other evidence from animal studies suggests that this reaction is catalyzed by DBH in synaptic vesicles within noradrenergic neurons in the brain.'"`UNIQ--ref-00000036-QINU`"''"`UNIQ--ref-00000037-QINU`"'
See also
- Hydroxynorephedrine
References
- ↑ '"`UNIQ--ref-00000029-QINU`"''"`UNIQ--ref-0000002A-QINU`"''"`UNIQ--ref-0000002B-QINU`"''"`UNIQ--ref-0000002C-QINU`"''"`UNIQ--ref-0000002D-QINU`"''"`UNIQ--ref-0000002E-QINU`"''"`UNIQ--ref-0000002F-QINU`"''"`UNIQ--ref-00000030-QINU`"'
References
- ↑ "p-Hydroxynorephedrine". NCBI. PubChem Compound. Retrieved 25 October 2013.
- 1 2 "Adderall XR Prescribing Information" (PDF). Medication Guide. United States Food and Drug Administration. Retrieved 7 October 2013.
- ↑ "Amphetamine: Biomedical Effects and Toxicity". NCBI. Pubchem Compound. Retrieved 12 October 2013.
- ↑ Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". J. Pharm. Biomed. Anal. 30 (2): 247–55. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
External links
- p-Hydroxynorephedrine at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.