This question is prompted by (comments at) another one. There, I was surprised to find that despite traditional claims to the contrary, Boltzmann himself did once write his formula $S=k\log W$:
$\hspace{11em}$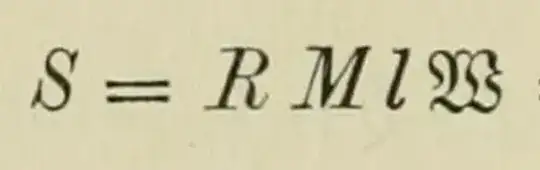
That’s in his book (1898, §61, p. 172), with a pointer to (1896, §8, p. 60) where he says the same thing in words, and emphasizes that $RM$ is the same constant for all gases. In fact, if we note that his $R$ is the specific gas constant (equal to $P\ /\ \rho T$ by the gas law, p. 53) and his $M$ the molecular mass $\rho V\ /\ N$, we see on multiplying that his $RM$ is indeed our $PV\ /\ NT=k$. So all seems well.
But now: there is another common story, which we get to hear again as 2018 is the year the kelvin should be redefined by freezing $k$. E.g. White and Fischer (2015) (emphasis mine):
Although Boltzmann published his famous definition of entropy in 1877, the constant of proportionality in Boltzmann’s definition, was not identified as Boltzmann’s constant until 1900 when Planck published his analysis of blackbody radiation (1900a, 1900b), where he identified the constant as $k$ and named it after Boltzmann.
And that, again, seems not true. Of course Planck does introduce $k$ — in (1900b, p. 241), after $h$, as “a second constant of nature” such that an entropy is $k\log\mathfrak R_0$. But he does not name it after Boltzmann there — nor, unless I missed it, in any of the obvious or oft-quoted places. Not in the follow-up articles (1901a, 1901b). Not in the Boltzmann Festschrift (1904: no mention of $k$). Not in his books (1906, 1910, 1913, 1930). Not in his Nobel lecture (1920):
This constant is frequently termed Boltzmann’s constant, although to the best of my knowledge Boltzmann himself never introduced it
Not in his autobiography (1948) where $k$ is “the so-called absolute gas constant”. And most remarkably — even if meant with self-deprecating irony — not in his recollections (1943):
I (...) was not even taken seriously, in some places. But I did not let such doubts deter me from trusting my constant $k$.
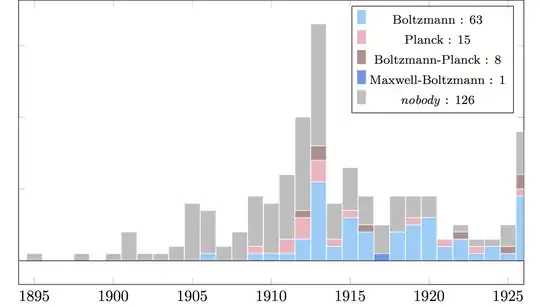
The literature also seems to have settled slower than legend has it — see chronology in the CW answer below. (A name Planck did propose in (1900b, p. 245) is “Boltzmann-Drude constant” for $\alpha=3k/2$, but few besides Abraham (1905, pp. 284, 362) seem to have adopted it — e.g. Perrin (1909) calls $\alpha$ “la constante d’énergie moleculaire”.)
So: If not in 1900, when did $k$ get its name? Was there ever a debate (e.g. after the unveiling of Boltzmann’s famous tombstone)? Was there a concerted decision? Were the above-quoted pages of Boltzmann’s book ever invoked? Or was it no-one’s doing, just resolution by attrition? Finally, if Planck did not name the constant after Boltzmann, how did we end up with the tale that he did?