Both of them.
The composition of the atmosphere, crust, mantle, core and bulk earth are all notably different.
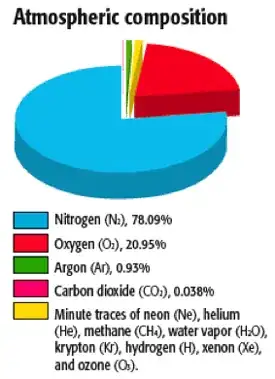
The atmosphere is composed of ~78% nitrogen and ~21% oxygen, with small amounts of other gases.
The bulk composition of the earth by weight is mostly, iron, oxygen, silicon and magnesium, in that order, with all the other elements making up only about 5% of the earth's weight. Most of the earth's iron is in the core, which is about 85% iron. The rest of the earth is dominated by oxygen and silicon, primarily in the form of silicate minerals, which consist of $\ce{SiO4^{4-}}$ tetrahedra linked in different ways and with different cations filling in the gaps.


