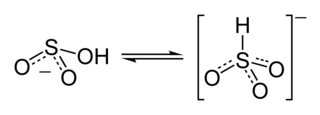
Hydroxy(phenyl)methanesulfonic acid, or benzaldehyde adduct is the product of reacting benzaldehyde with sodium metabisulfite ($\ce{NaS2O5}$) solution. It is obviously a well-known and often used reaction, and yet, I'm not able to find much info on the adduct, or the reaction itself.
One thing I managed to dig out is its corresponding PubChem entry.
I want to find out the exact mechanism of the reaction with an equation, a bunch of videos of someone performing it will be nice too...
But most importantly - the hydroxy(phenyl)methanesulfonic acid: What is its color, texture and so on? What is its crystalline structure? Is it supposed to smell of benzaldehyde, how soluble it is in different solvents, how should it be stored, is it harmful or dangerous, does it decompose in some conditions, melting point, and so on.