Are phenols good antioxidants? If so what makes them good? I think it is because they oxidize easily but I'm not sure how that helps.
Asked
Active
Viewed 126 times
1
-
https://chemistry.stackexchange.com/questions/85486/how-do-antioxidants-work https://chemistry.stackexchange.com/questions/62729/what-makes-a-good-antioxidant – Mithoron Apr 11 '18 at 20:39
-
https://chemistry.stackexchange.com/questions/16088/what-has-stronger-antioxidant-potential-bht-or-bha – Mithoron Apr 11 '18 at 20:44
1 Answers
4
Phenolic compounds, or in general aromatics actually, has electrons which many oxidants can easily abstract or bind to molecule.
From the above link in page 17,
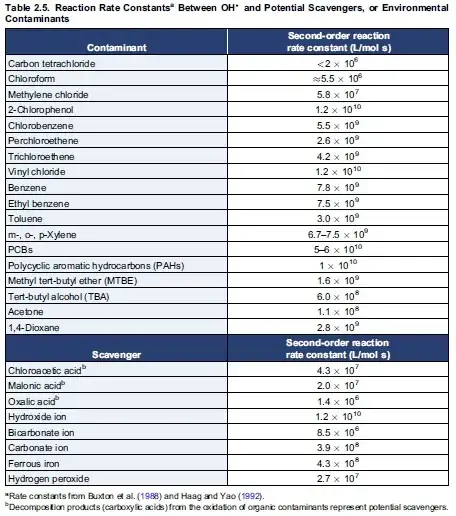
as you see the highest rates, order of magnitude or even more higher ones come from aromatics, or at least pi-bond between carbons. It can reasonably assumed that if you have these compounds in a mixture, the possibility of any oxidant introduced reacts with them is higher than the other ones. In short, they are good anti-oxidants in that way.
Güray Hatipoğlu
- 1,019
- 7
- 21