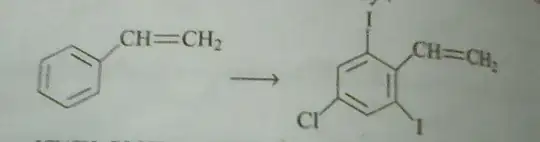
The question is basically to figure about the steps for the following transformation.
Since ethene is a electron donating group and weakly ortho para directing so it makes electrophilic substitution difficult.hence we must add bromine water($\ce{HOBr}$) to the double bond.After this if we add $\ce{Cl2}/Fe$ we will get ortho and para positions substituted by chlorine.However I am facing difficulty changing the ortho chlorine atoms to iodine.Please suggest me a way to proceed.Thanks.