So I was looking at factors that control the relative strengths of acids and bases.
We know that $\ce{HI}$ is a stronger acid that $\ce{HBr}$ because $\ce{I}$ is a much larger atom.
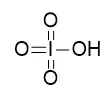
But then, I read somewhere that $\ce{HBrO4}$ is stronger than $\ce{HIO4}$, because in this case it's electronegativity that plays the important role.
How do you know whether to use size or EN as a guideline for comparing strengths of acids/bases?