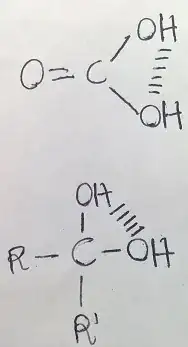Is an intramolecular hydrogen bond possible in such compounds?
What are the requisites to have intramolecular hydrogen bonding?
- 44,013
- 13
- 159
- 319
- 393
- 2
- 6
- 11
-
In second one the two R groups are +I group as well as steric hindering hence drastically reduces stability, also there is repulsions due to lone pairs on oxygen. – Sujith Sizon Jan 04 '16 at 05:07
2 Answers
Is this possible?
Probably not possible. An elementary explanation would be "too much ring strain."
https://en.wikipedia.org/wiki/Ring_strain
I'm a bit confused: What are the requisites to occur a intramolecular hydrogen bonding?
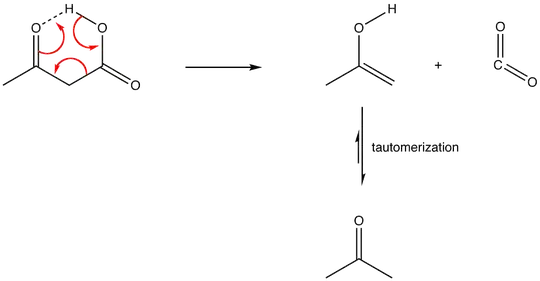
5 and 6-membered rings are more favorable. As a matter of fact, beta-ketoacids, when warmed, readily decarboxylate. Why? The intermediate to decarboxylation is stabilized through hydrogen bonding.
Note the 6-membered ring in the picture below.
- 18,815
- 49
- 177
- 341
As the intramolecular H-bonding is absent in both cases.
Carbonic acid
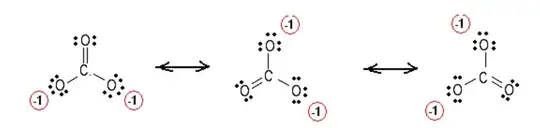
In aqueous solution of carbonic acid is a weak acid. It dissociates into its constituent ions as carbonate ions and hydrogen ions. Now, the CO3-2 ion, is stabilized by resonance and produce three equivalent resonating structures.
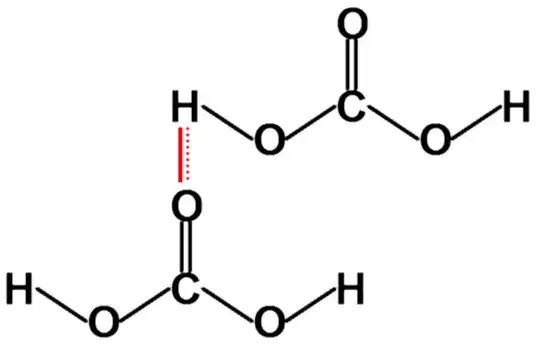
This makes carbonate ion more stable rather than through intra molecular H-bonding. Now, I already said that carbonic acid is weak acid means all the molecules of carbonic acid are not dissociated. The molecules of carbonic acid which remain undissociated form inter-molecualr H-bonding with each other.
One thing that carbonic acid does not exist via intra-molecular H-bonding because, there is too much ring strain.
Gem-diols
In gem-diols there are two -OH groups present on a single carbon. You can get detail reason that why intra-molecular H-bonding is absent in gem-diols.
There is a common example of gem-diol where intra molecular H-bonding is present is chloral hydrate.
- 969
- 1
- 5
- 14
-
Welcome to Chemistry.SE! The dissociation $$\ce{H2CO3 (aq) <=> CO2 (aq) + H2O}$$ is indeed the predominant reaction, favouring carbon dioxide. That is one of the main reasons, why many carbonates readily decompose at low pHs. || Mathematical expressions and equations can be formatted using $\LaTeX$ syntax. If you want to know more, please also have a look here. – Martin - マーチン Jan 04 '16 at 10:41