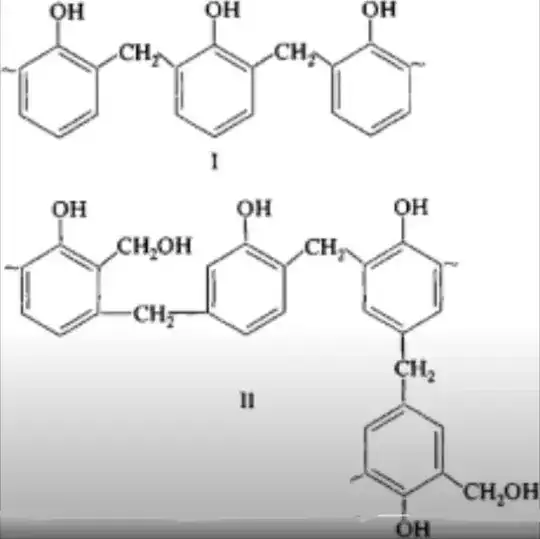
The following chemical structure is phenolic resin. How is this chemical made?
Asked
Active
Viewed 102 times
1 Answers
1
Here is an experiment that all my students have carried out in the lab.
Make a solution with about $3$ mL $40$% solution of methanal and an equal volume of phenol. Transfer the solution into a small paper cup. Add a equal volume of concentrated $\ce{HCl}$, when stirring with a glas rod. This produces a strong exothermic reaction, and the result is the described phenolic resin, whose common name is bakelite.
Maurice
- 28,241
- 3
- 29
- 61
-
The phenolic resin called bakelite is rather a phenolformaldehyde resin. – Poutnik Mar 14 '21 at 17:45
-
My synthesis is producing a phenol formaldehyde resin, as methanal is also called formaldehyde, – Maurice Mar 14 '21 at 17:47
-
Yes, that is correct. You have initially written methanol, what is understandable as easily done typo. More exactly, it would be rather 40%(probably) solution of methanal, which is itself gaseous. – Poutnik Mar 14 '21 at 18:26